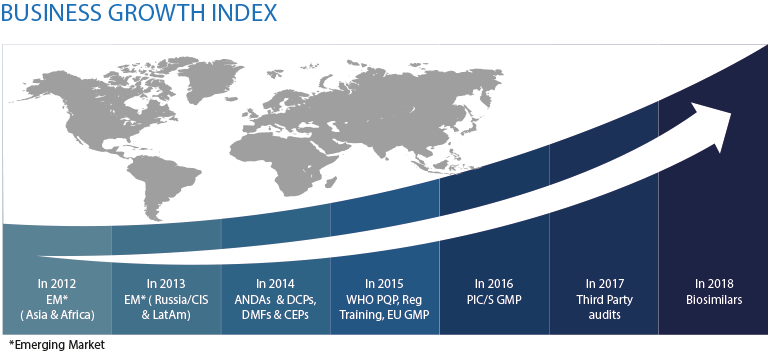
Established in 2012, Metina is an India based company with its headquarters in Mumbai and subsidiaries in Singapore, Malaysia and Australia.
Metina is a pharmaceutical and bio-pharmaceutical consultancy organization engaged in GMP consulting, Regulatory services for small molecules & biosimilars, Third Party QP Audits, Technical Due-Diligence projects & Portfolio Strategy. Our services span Regulated as well as Emerging Markets.
Our technical team comprises of skilled resources with core bio/ pharmaceutical industry experience having in-depth and updated understanding of GMP, Regulatory Affairs, Portfolio Strategy, Product Development and Project Management.
We strive to be a reliable Regulatory, GMP, Due-Diligence partner to the industry by offering comprehensive, knowledge driven, time bound and high quality services.